
Mega counts with an
abnormal colony morphology. In these colonies cells were smaller and the number
of eetls per colony was mueh higher than in normal CFU-Mega derived colonies. In
addition to the patient we tested earlier,6 we were ahle to test the
presence of CFU-Mega in the bone marrow From orily one TAR patient, a newborn
baby-gin whose bone marrow was aspirated for diagnostic purposes.
We could not detect any
megakaryocyte colony growth after stimulation with TPO, indicating that
megakaryoeyte devel opment is markedly irnpaired. Ilowever, more patients liave
tu be tested to malte a final statement conceming megakaryo eytic colony growth
in vitro. In contrast, the numbers of nonmegakaryocytic colonies (CFU-GM and
BFU-E) stimulated by other growth factors (G-CSF GM-CSF IL-3 SCF

and EPO) were
increased. This is in aeeordance with reports of episodes of leukocytosis and
leukemoid reaetions with white cell counts greater than 35,000/ÁL and a left
shift of myeloid eells in infants with TAR syndrome.2~3 These reie
tions eould be caused by elevated leveis of other hematopoi efle growth faetors.
In fact, we found elevated levels of IL 11 in at least three out of five
patients affected with the TAR syndrome. IL- 11 is a cytokine that is reported
tu promote the growth of myeloid and erythroid precursors. 34 However,
elevation of this cytokine also could have occurred indepen dent of
thrombocytopenia seen in these patients.
Platelet
funetions were reported tu be normal in patients with TAR syndrome,3 although
few patients had ahnormal platelet aggregation and storage pool defects.
8,9 Beeause of the high blood volume required in testing platelet aggrega
tion horn thrombocytopenic patients. we only measured the expression of the
activation-dependent antigen CD62P on platelets after stimulation with ADP or
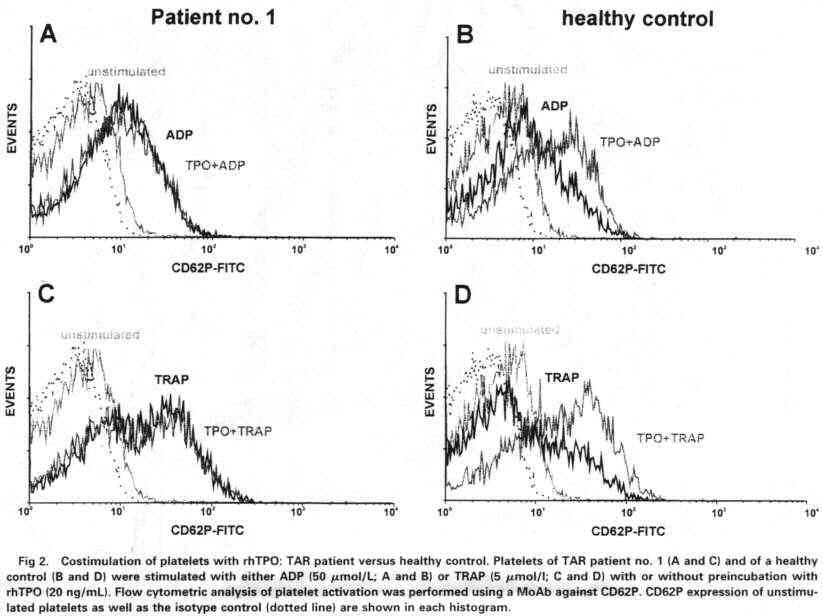
TRAP. Under these experimental conditions. we found normal platelet activation
in all TAR patients tested.
We examined
the in vitro reactivity of platelets tu TPO. Although TPO by itself does not
cause an inerease in a- granule seeretion as assayed by CD62P expression. it
upregulates the response tu ADP or thrombin receptor ago nists.27~35~36 However,
TPO by itseif causes signifleant tyro sine phosphorylations in a number of
different proteins in platelets.21~35~37 In contrast, platelets from
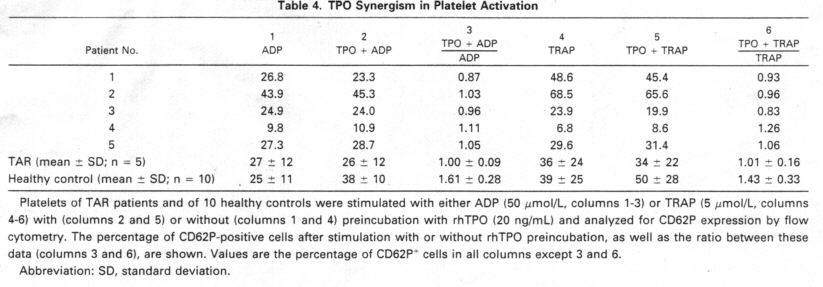
TAR patients showed no or very little reaetivity tu TPO. We found no synergism
of TPO tu the platelet aetivators ADP and TRAP. Furthermore there was no (3 of
4) or very wealt (1 of 4) additional tyrosine phosphorylation of platelet
proteins after stimulation with TPO. From this defeetive reactivity of plate
lets in response tu TPO we assume a defeet of all cells of the
megakaryocytopoiesis ineluding megakatyocyte progenitor cells. This defeetive
response might be the cause ofthrombo
cytopenia in the TAR syndrome. Signal transduction path ways downstream of c-Mpl should be essentially similar in platelets and their progenitors in the bone marrow. Regardless of whether the signal transduction in platelets is a simple remnant horn the megakaryocytes or whether there is a phys iological role of TPO in platelet activation. Miyakawa et a121'37 showed phosphorylation of the signal transduction molecules Jak2, Shc, Stat3. and Stat5 in platelets in response to TPO. These data correspond tu those obtained with cell (ines normally expressing or engineered tu express c Mpl.22.23 38. We could show that c-Mpl is expressed on the surface of platelets horn TAR patients in similar amounts as in healthy controls. In addition the molecular weight of the receptor corresponds tu that of normal donors. However. puint muta tions in c-Mpl of TAR patients cannot be excluded as a cause for the defeet in signal transduction. This seenarirt resembles very much the situation in congenital neutropenia patients in which we could show elevated G-CSF serum levels and normal G-CSF receptor expression but defeetive response tu G-CSF in vitro and in vivo.39 Nevertheless, cnn genital neutropenia patients responded weIl tu pharmacologi cal dosages of G-CSF with an inerease of neutrophils associ ated with a reduetion of bacterial infeetions. suggesting that treatment of TAR patients with rhTPO might help tu inerease platelet numbers and improve elinical problems associated with thrombocytopenia.
ACKNOWLEDGMENT
We thank the foHowing physirians for providing us wuh samp(es from their
TAR patients: Dr H. Habenicht (Hamburg. Gennany). Dr H. Jakobi, Dr H.-P.
Haastert (Celle, Germany). Dr R. Burghard (Memmingen, Germany), Dr H. Schmidt.
Dr U. Richter (Detmold. Gennany), and Dr E. Koscielnak (Stuttgart, Germany). In
addition we are grateful tu Dr Michael Martin, Department of Molecular
Phannaco(ogy. MHH. for performing the IL-6 and IL-1 1 ELISAs and Dr Muhan Huang
for perfonning the LIF ELISA.
REFERENCES
(. Greenwald HM. Sherman 1: Congenital essential thrumhocyto penia. Am J
Dis Child 38:1242, 1929
2. Hall JG, Levin J, Kuhn
JP, Ottenheimer EJ. van Berkum KAP.
McKusick VA: Thromhocytupenia with absent radius (TAR).
Medi eine 48:411,1969
3. Hedherg VA, Lipton JM: Thrumhucytupenia with absent radii. A review of
100 cases. Am J Pediat Hematol Oneol 10:51, 1988
4. Romans AC, Cohen JL, Mazur EM: Defeetive megakaryucytu puiesis in the
syndrome of thrumhocytopenia with absent radii. Br J Haematnl 70:205, 1988
5. Michalevicz R, Baron 5. Burstein Y: Ostenelast-like cells grow in
cultures of multipotent hematnpoietic progenitnrs in thromhocytn penia and
absent radil (TAR) syndrome. Israel J Med Sei
24:42. 1988
6. Kanz L, Kostielniak 13, Welte K: Colony-.stimulating activitv (CSA)
unique for the megakaryncytic hetnopoietic cell lineage. pres ent in ihe plasma
of a patient with the syndrome of thromhocytnpenia with absent radii (TAR).
Blond 74:248a, 1989 (suppl 1. ahstr)
7. de Alareon PA, Graeve JA, Levine RE. McDonald TP. Beal DW:
Thrnmbncytnpenia and absent radii syndrome: Defeetive mega
karyncytnpniesis-thrnmhncytnpniesis.
Am J Pediat Hematol Oneol