
The TAR syndrome is a rare
eongenital disorder eharaeter ized by the absenee of radb on both arms and
severe throm boeytopenia during the first years of life. Reeent studies showed
that the thromboeytopenia seen in TAR patients is eaused by a defeet in the
megakaryocytopoiesis/thromboey topoiesis.4~7 Different possible
meehanisms for this bone marrow failure ean he postulated: (1) absenee of
humoral or eellular stimulators of megakaryoeytopoiesis, (2) absenee of
megakaryoeytie progenitor eells, (3) eellular defeets in megakaryoeytie
preeursors (eg, reeeptor defeets), or (4) pres
Fig 1. Expressicn of c-MpI on piatelets from TAR patients. IAI c-MpI expression on the surface of plateiets es measured by flow cytometry see Materials and Methodsl. Grav. isotype Gontrol; black. anti c-Mpi. IB Detection of c-Mpl in pletelet lysates after immunoprecipitetion and Western blot analysis with anti-c.Mpl entibody Ml lchemo luminescence detection, See Meterjeis and Meth odsl. Lene 1, heelthv donor; lene 2, TAR patient na. 1.

enee of humoral or edlular
inhibitors of megakaryoeyto poiesis.
There are eontroversial
reports eonceming Mega-CSA or “TPO-like" aetivities in the sera of TAR
patients. Our group6 and others4 found high Mega-CSA in
the sera of TAR patients. However, de Alareon et a17 reported
Mega-CSA and “TPO-like" activities in the serum of one TAR patient. whieh
were within the range of normal and below the high levels seen in
amegakaryoeytie subjects. Miehaleviez et a15 even found an inhibitory
effeet of plasma of one TAR patient on the growth of multipotent and
megakaryoeytie progeni tors. After the diseovery of TPO and its reeeptor e-Mpl
we were able to measure the aetivity of this major humoral regulator of
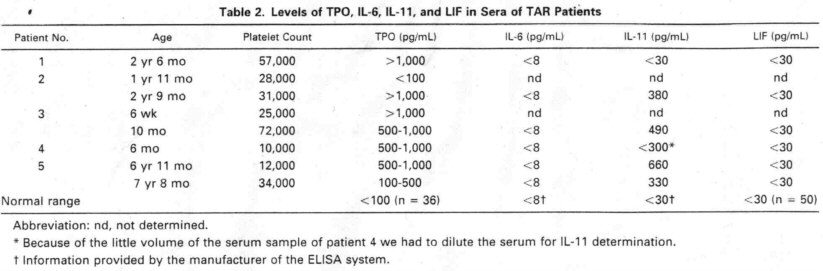
megakaryoeytopoiesis and thrombocytopoiesis in the sera of TAR patients. We
found elevated levels of bioaetive TPO in sera from all TAR patients tested,
exelud ing a TPO produetion defeet as the eause of thrombocyto penia in TAR
syndrome. lt is a eommon phenomenon that
lineage speeifle
hematopoietic growth factors are inversely regulated with the number of
eorresponding mature eells in peripheral blood. This has been shown for G-CSF,28
EPO.29,30. and TPO31,32 and was found to be the ease
in a number of eongenital eytopenias as the severe eongenital neutropenia. also.33
The first determination of TPO serum levels of patient 2 yielded no
elevated level of this eytoldne. We have no explanation of that diserepancy.
However, the first determi nation has been made with a relatively old serum
sample and we eannot exelude a loss of activity during inappropriate storage. On
the other hand, variations of TPO levels in TAR syndrome might be the reason for
differenees in former re ports.
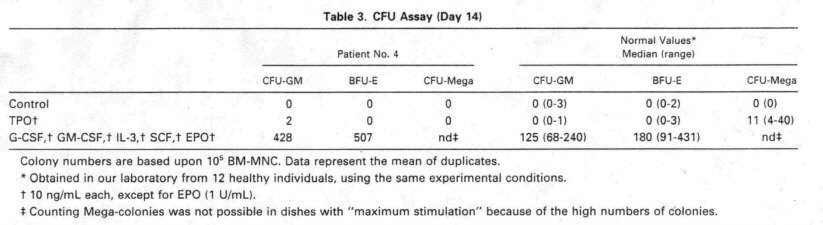
The presence of eolony
forming units megakaryoeyte (CFU-Mega) in the bone marrow of TAR patients is
another pomt of eontroversy in the literature. Whereas most groups, like us, did
not find any growth of megakat'yocytic eolonies from TAR patients.4'6
de Alareon et a17 showed normal CFU
the c-Mpl on the platelets
from the TAR patients was found tu be 86 kD as determined bv SDS-PAGE and was
not different from that of healthy controls (Fig 1B).
Costiruulotjou of
p/atelets with TPO.
TPO
svnereizes with ADP or thrombin receptor agonists to activate platelets as
measured by increased expression of P-seleetin (CD62P(.2 We used this
fact for in vitro testing of TPO reaetivitv of platelets from TAR patients.
(CD62P).˛7 served as an aetivation-dependent marker. Using this method,
platelets from normal individuals showed a synergism of rhTPO (5 - 10 ng/mL) with
the platelet aetivators ADP and TRAP. AI though there was a great variety
in the reacüvity of platelets tu the activators ADP and TRAP, platelets
from all TAR patienis showed reactivity in the range of normal controls (Fig 2
and Table 4). However, we eould not deteet the ex peeted synergism of these
platelet aetivators with rhTPO up to enneentrations of 1 µg/mL in these
patients. The results of one representative expetiment are shuwn in Fig 2. To
quantitate the synergistic effeet of TPO on platelet activation we ealeulated
the ratio between the amount of CD62P-posi tive eells afier TPO costimulation (with
ADP or TRAP) and the amount of CD62P-positive eells after control stimulation
without TPO (Table 4). TPO preineubation (20 ng/mL) lead tu a l.6-fold
(~0.3)
enhaneement of ADP stimulation (50 µmol/L) or 1 4-fold
(~0.3)
enhaneement of TRAP stimula tion (5 µmol/L) in platelets of normal individuals
(n = 6). In enutrast, TPO stimulation indiees in TAR patients were
1.0 0.1 for ADP
and 1.0 0.2 for TRAP. respeetively
(Table 4).
Signa/ transducrior of
c-Mpl after TPO binding. Becemse platelets express e-Mpl they are used as a mode for investigating
signal transduetion pathways of TPO. c-Mpl is a member of the cytokine reeeptor
superfamily, whieh leads tu tyrosine phosphorylation of eellular proteins.
We could show that
stimulation of platelets with hieb enneentrations of rhTPO for 5 minutes indueed
phosphoryla tion of several proteins, espeeially at 86 kD and higher (Fig 3).
Stimulation with only 50 ng/mL rhTPO lead tu phosphor vlation of a 1 l0- protein
(data not shown). In Contrast, platelets from three nut of four TAR patients
(no. 1,2. and 5) ineubated wuh rhTPO enneentrations up tu 12 µg/mL showed no
difference in tyrosine phosphorylation as eom pared with unstimulated eells (Fig
3), one other patient (no. 3) showed a weak phosphorylation of the 1 l0-kD
protein oniv after stimulation with rhTPO enneentrations hieher than 5 µg/mL.
DISCUSSION